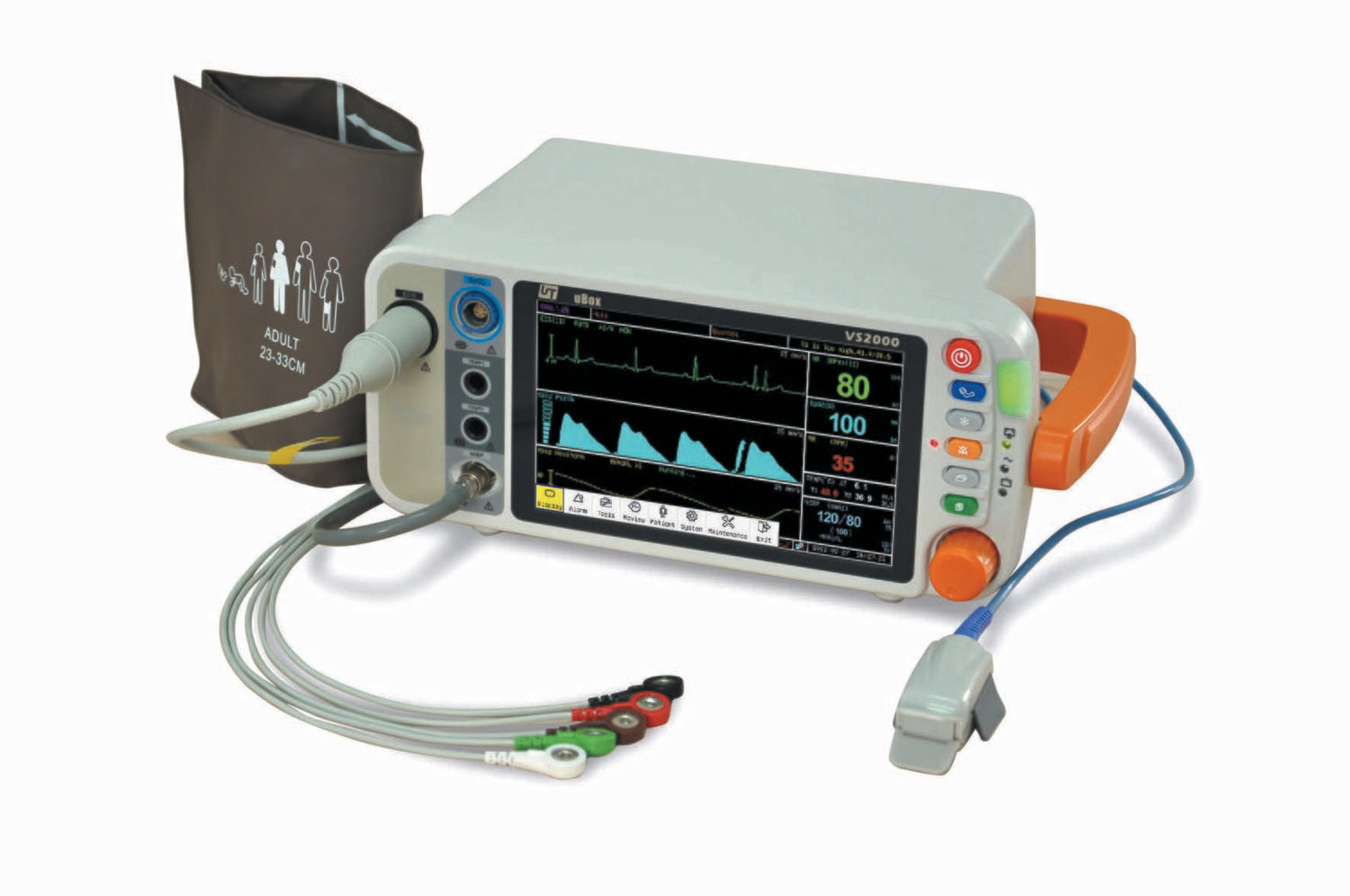
VS2000 7" Vital Signs Monitor - ECG RESP SpO2 NIBP TEMP PR
The VS2000 vital signs monitor is intended to be used in special procedure labs and other areas of a hospital or clinic where patient monitoring systems are needed. The standard parameters includes 5/3 leads electrocardiography(ECG), non-invasive blood pressure (NIBP), pulse oximetry (SpO2), Respiration Rate(RR), temperature(TEMP), optional parameter include ETCO2.
Main Features
- 7.1' coloured and bright, high definition (800 x 480) TFT LCD screen;
- Parameters: SpO2, ECG, Heart Rate,NIBP, TEMP*2, RESP (Optional: ETCO2)
- Suitable for Adult, Padiatric, Neonate ;
- 72 hours graphic and tabular trends of all parameters;
- 32 seconds full-disclosure waveform review
- 500 NIBP and SpO2 measurement data can be stored and recalled;
- 3-level audible and visual alarms and alarm events can be stored and reviewed.
- 100M Base-T Ethernet Interface, Support to connect to IPMS8000 Patient Management System;
- Sidestream technology accommodates intubated and non-intubated patients
- Ergonomically designed handle to fit your hand comfortably
- Can link with IPMS8000 intelligent patient monitor system
- Multi-language: English, Chinese, French, Spanish, Germany
Standard Package:
- VS2000 main unit(ECG+NIBP+SpO2+RESP+TEMP+PR)
- Adult Finger Clip SpO2 sensor
- 5-lead ECG cable
- 5 ECG Clips
- NIBP cuffs for Adult
- NIBP Extension Tube
- Temp Rectal Probe
- Lithium Battery
- Power cord
- User Manual
Optional Accessories:
- Central Monitoring Software
- Mobile Trolley
- Carrying Bag
- Mainstream or Sidestream ETCO2 Sensor and Accessories
Warranty:
Main unit: 2 years
Accessories: 6 months
| Technical Specification |
|||||
| Enclosure |
|||||
| Weight |
2.05 kg (4.52lbs) |
||||
| Dimensions |
32.4cm × 12.9cm × 18.3cm |
||||
| Display |
7.1-inch diagonal high-resolution TFT (Thin Film Transistor) Active Matrix LCD 800 X 480 |
||||
| Alarms |
|||||
| Categories |
Patient status and system status |
||||
| Notification |
Audible and visual |
||||
| Setting |
Full storage, low battery and sensor off |
||||
| Range |
Pulse rate (30-350bpm), SpO2 (0-100%), NIBP (0-300mmHg), RESP (5-150rpm), TEMP (24.5-45.5℃) |
||||
| Alarm volume level |
45dBA to 85 dBA at 1 meter distance (adjustable) |
||||
| Range, Accuracy and Resolution |
|||||
| Range Type |
Range Values |
||||
| Measurement Ranges |
|||||
| SpO2 |
0-100% |
||||
| Pulse rate |
30-350bpm |
||||
| NIBP |
Systolic:40 to 270 mmHg Mean Arterial:30 to 220 mmHg Diastolic:20 to 200 mmHg |
||||
| RESP |
0-150rpm |
||||
| TEMP |
0-50℃ |
||||
| Measurement Accuracy |
|||||
| SpO2 |
70-100% (±2%), <70% undefined |
||||
| Pulse rate |
±2bpm/±2% take maximum |
||||
| ETCO2 |
0~40mmHg ±2mmHg, 41~150mmHg ±10% |
||||
| TEMP |
±0.2℃ |
||||
| RESP |
±1rpm |
||||
| Resolution |
|||||
| Display |
800 X 480 |
||||
| SpO2 |
1% |
||||
| ETCO2 |
0.1mmHg |
||||
| TEMP |
0.1℃ |
||||
| RESP |
1rpm |
||||
| Pulse rate |
1bpm |
||||
| Electrical |
|||||
| AC power requirement |
100-240VA, 50/60Hz |
||||
| Power comsumption |
60VA,FUSE T1.5A |
||||
| Batteries |
|||||
| Types |
Lithium-lon Batteries |
||||
| Operating time |
≥4hours |
||||
| Charge time |
≤4hours |
||||
| Environment |
|||||
| Operating tempearture |
0-50℃ |
||||
| Storage temperature |
-40-75℃ |
||||
| Operating humidity |
15-95% |
||||
| Storage humidity |
10-95% |
||||
| Operating altitude |
-500-5000m |
||||
| Standards and Certification |
|||||
| IEC 62366 |
Medical devices-Application of usability engineering to medical devices |
||||
| IEC 62304 |
Medical device-software life cycle processes |
||||
| MDD 93/42/EEC |
European Medical Device Directive |
||||
| ISO 13485 |
Medical equipment-Quality management system |
||||
| EN ISO 14971 |
Medical devices-Risk management |
||||
| EN 60601-1 |
Medical electrical equipment |
||||
| EN 60601-2 |
Medical electrical equipment |
||||
| EN 60601-8 |
Medical electrical equipment |
||||
| EN 980 |
Symbols for use in labeling of medical devices |
||||
| EN 1041 |
Information supplied by the manufacture of medical devices |
||||
| ISO 80601-2-61 |
Medical electrical equipment-Particular requirements for the basic safety and essential performance of pulse oximeter equipment for medical use |
||||
| EN ISO 10993-1 |
Biological evaluation of medical devices |
||||
| EN ISO 10993-5 |
Biological evaluation of medical devices |
||||
| EN ISO 10993-10 |
Biological evaluation of medical devices |
||||
| ISO 14155-1 |
Clinical investigation of medical devices |
||||
| CE |
CONFORMITE EUROPEENNE |
||||
| SFDA |
State Food and Drug Administration |
||||